

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin D (Homo sapiens (Human)) | BDBM29806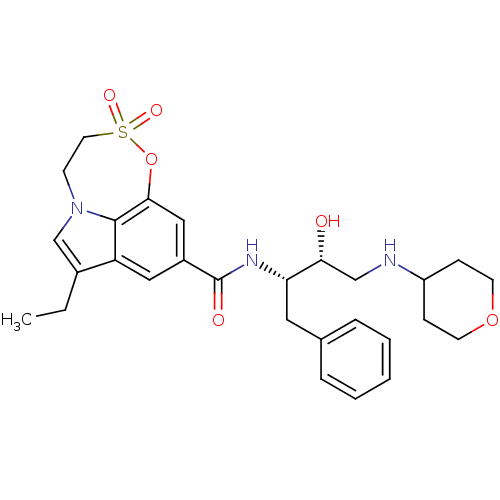 (sulfone tricyclic analogue, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29792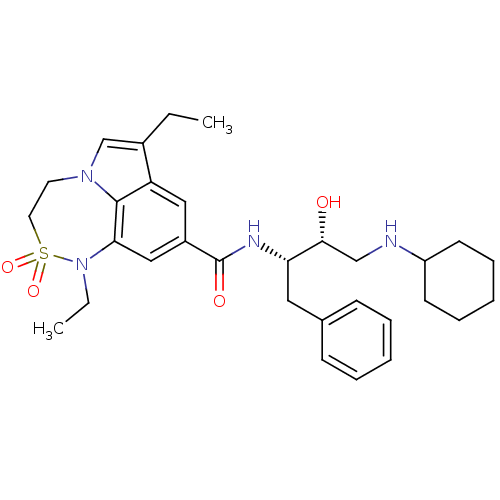 (7,6,5 tricyclic sulfonamide, 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29784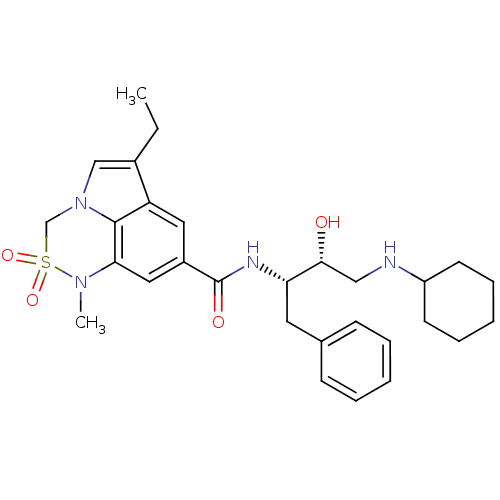 (6,6,5 tricyclic sulfonamide, 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29796 (sulfonamide tricyclic analogue, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29760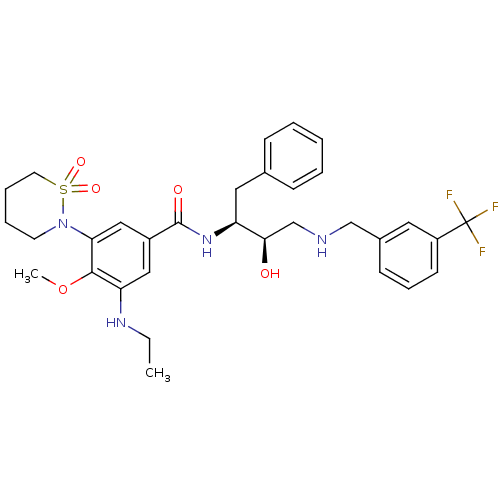 (hydroxyethylamine derivative, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29770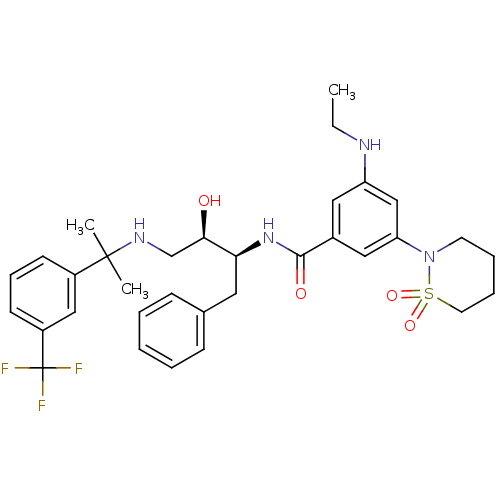 (hydroxyethylamine derivative, 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26508 (BMCL193669 Compound 26 | N-[(2S,3R)-4-(cyclohexyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26508 (BMCL193669 Compound 26 | N-[(2S,3R)-4-(cyclohexyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29783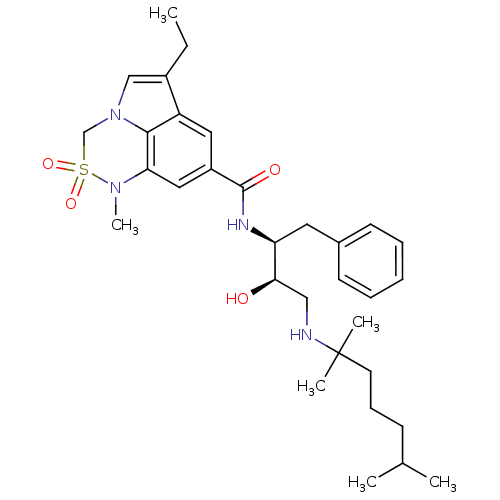 (6,6,5 tricyclic sulfonamide, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29777 (7,6,5 tricyclic sulfonamide, 15 | BMCL193674 Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29777 (7,6,5 tricyclic sulfonamide, 15 | BMCL193674 Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29759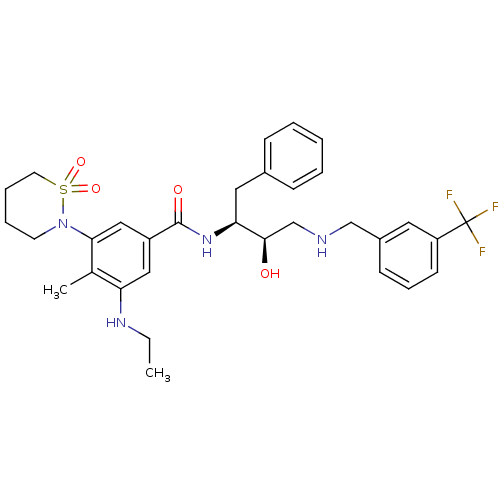 (hydroxyethylamine derivative, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26506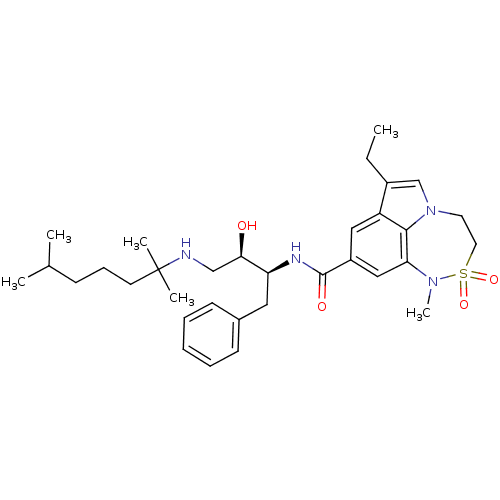 (BMCL193669 Compound 24 | N-[(2S,3R)-4-[(2,6-dimeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26506 (BMCL193669 Compound 24 | N-[(2S,3R)-4-[(2,6-dimeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29781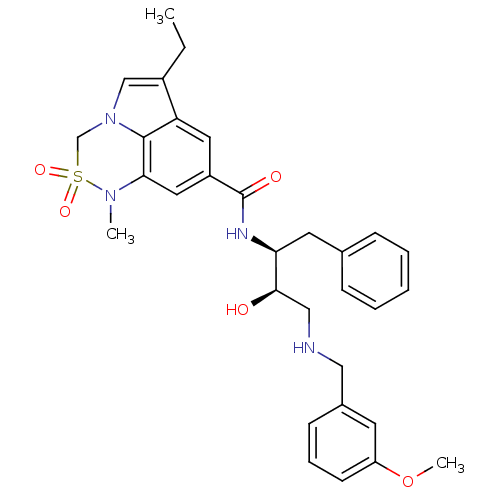 (6,6,5 tricyclic sulfonamide, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29778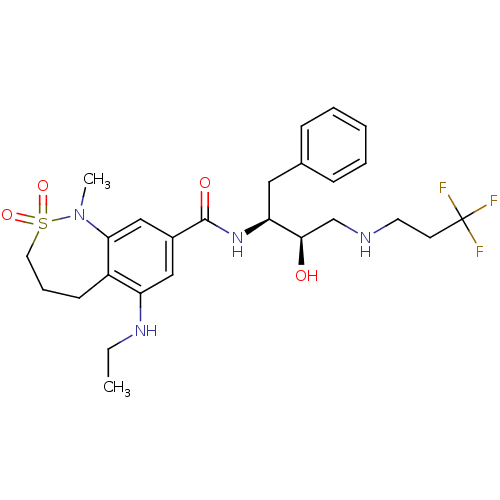 (bicyclic sulfonamide analogue, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26779 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26779 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM10145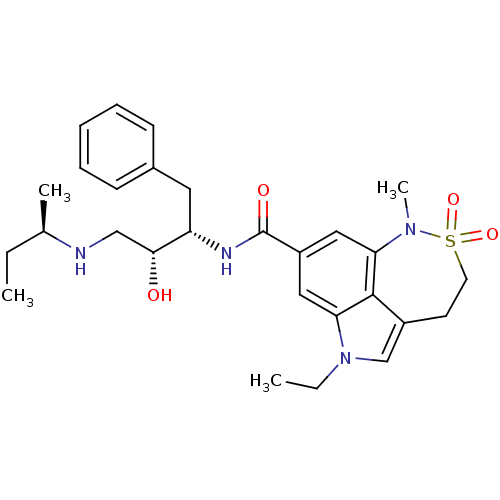 (lysine sulfonamide analogue 9 | methyl N-[(1S)-1-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29773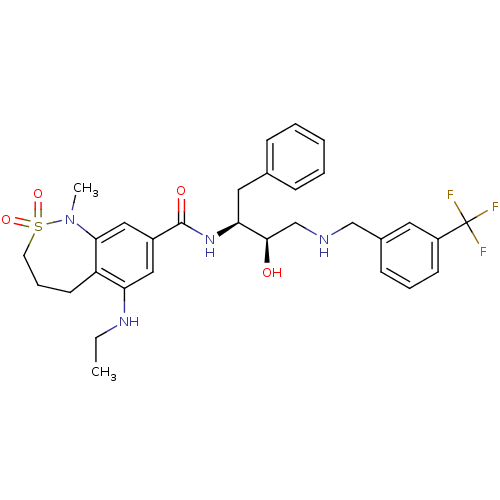 (bicyclic sulfonamide analogue, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26503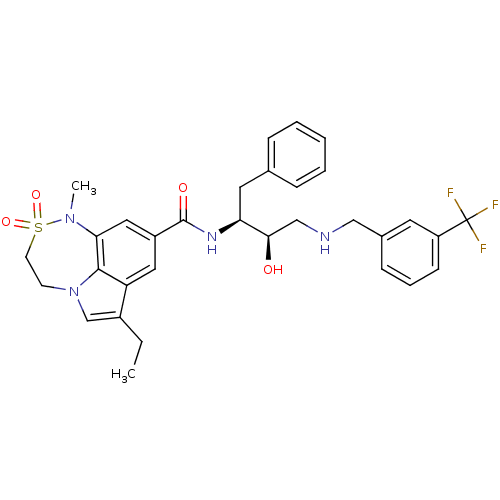 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26503 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26503 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29786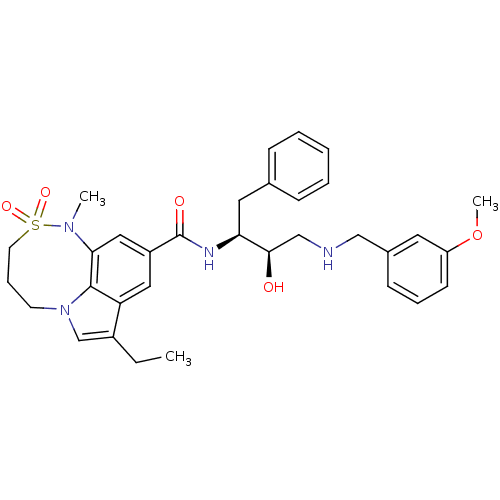 (8,6,5 tricyclic sulfonamide, 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29789 (7,6,5 tricyclic sulfonamide, 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26769 (3-ethoxy-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26771 (3-(2-cyanophenyl)-5-ethoxy-N-[(2S,3R)-3-hydroxy-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29794 (sulfonamide tricyclic analogue, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29805 (sulfone tricyclic analogue, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29772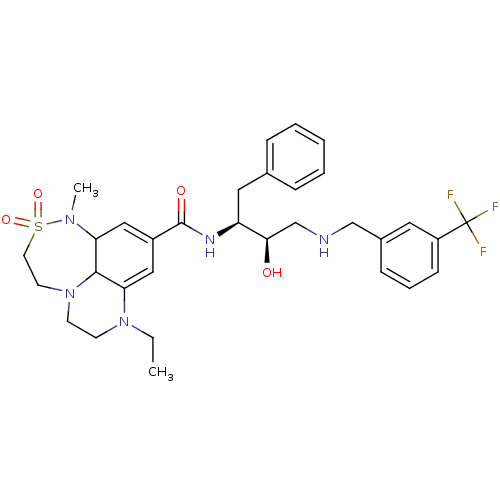 (7,6,6 tricyclic sulfonamide, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26509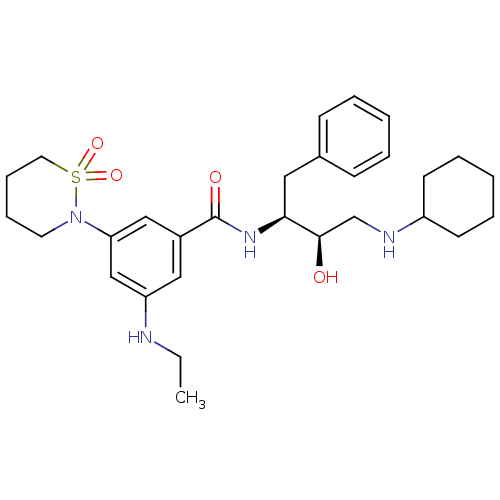 (N-[(2S,3R)-4-(cyclohexylamino)-3-hydroxy-1-phenylb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26782 (N-[(2S,3R)-1-(3,5-difluorophenyl)-3-hydroxy-4-{[(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26777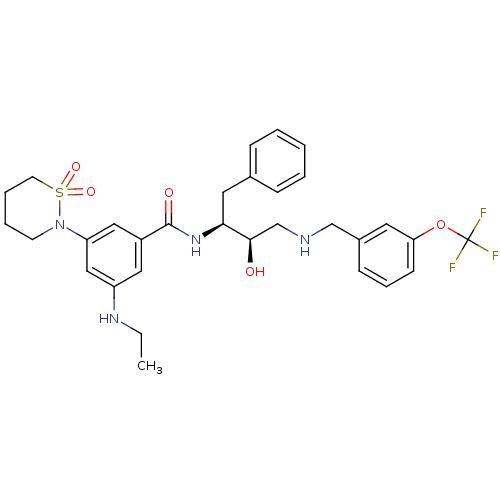 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26788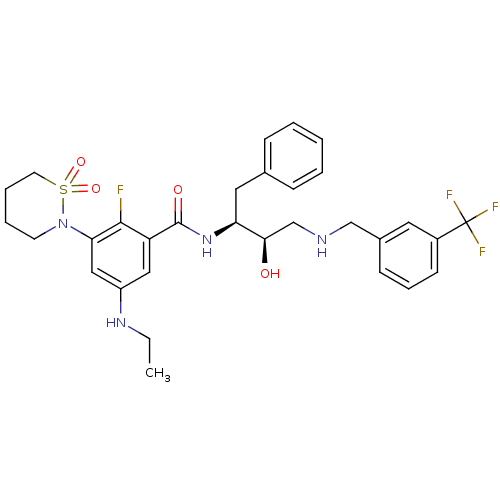 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29787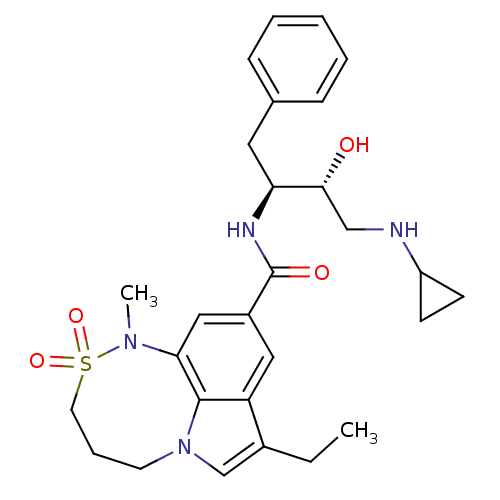 (8,6,5 tricyclic sulfonamide, 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26770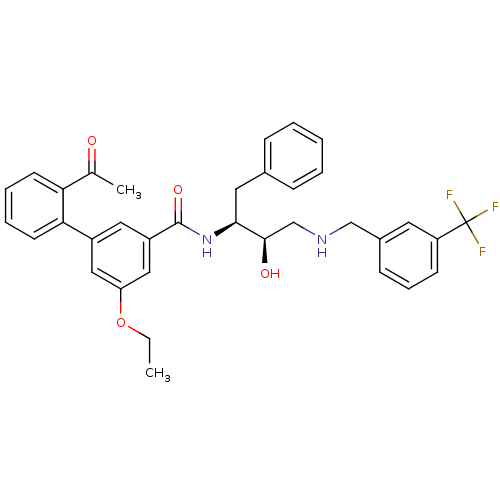 (3-(2-acetylphenyl)-5-ethoxy-N-[(2S,3R)-3-hydroxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29782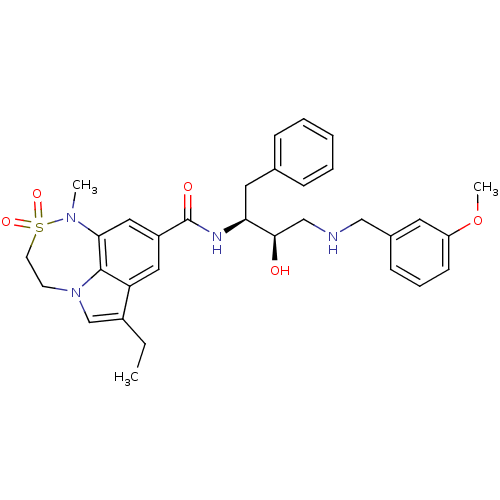 (7,6,5 tricyclic sulfonamide, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29790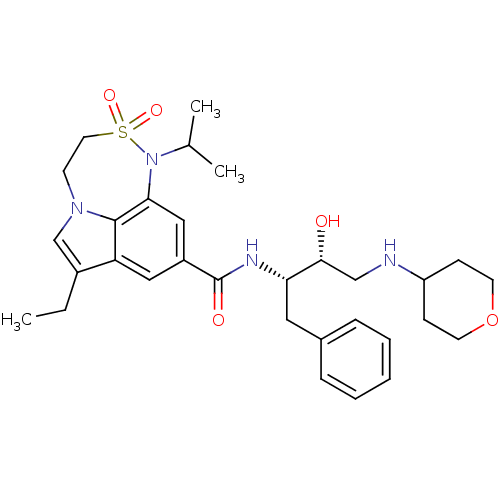 (7,6,5 tricyclic sulfonamide, 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29808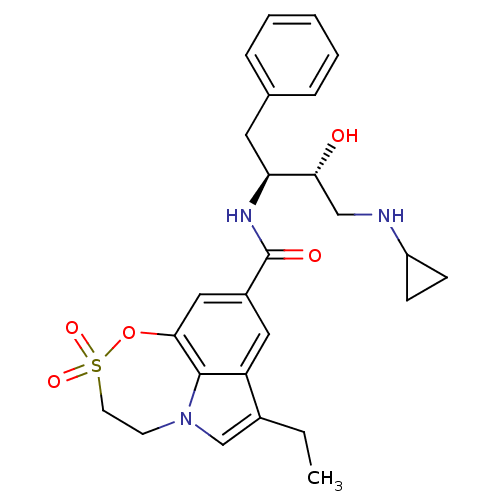 (sulfone tricyclic analogue, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26778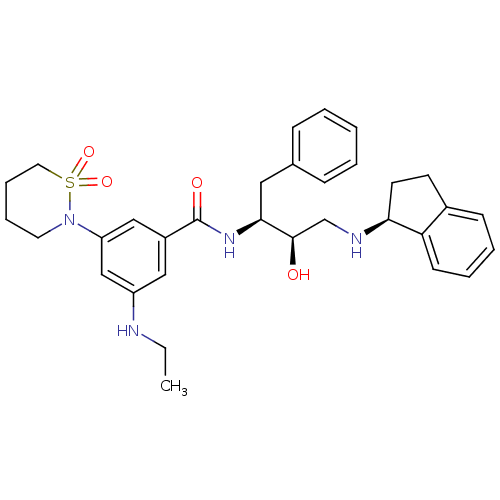 (N-[(2S,3R)-4-[(1S)-2,3-dihydro-1H-inden-1-ylamino]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29766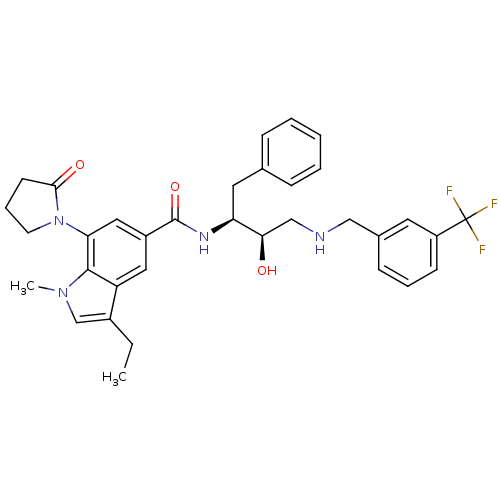 (hydroxyethylamine derivative, 18b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29758 (hydroxyethylamine derivative, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29763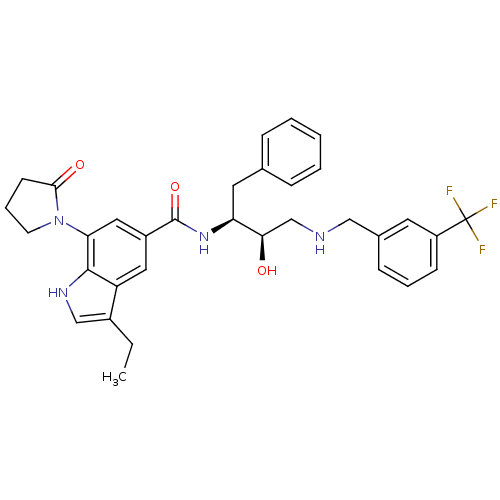 (hydroxyethylamine derivative, 18a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29776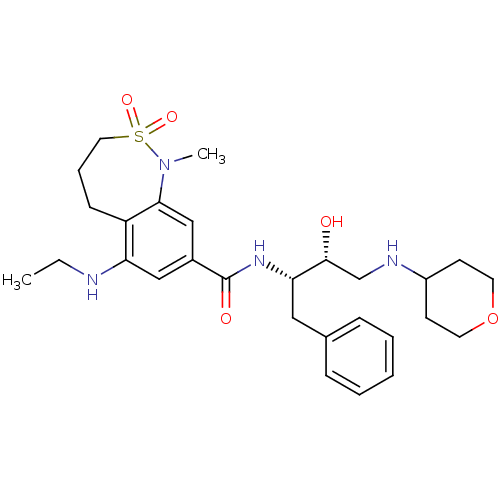 (bicyclic sulfonamide analogue, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29757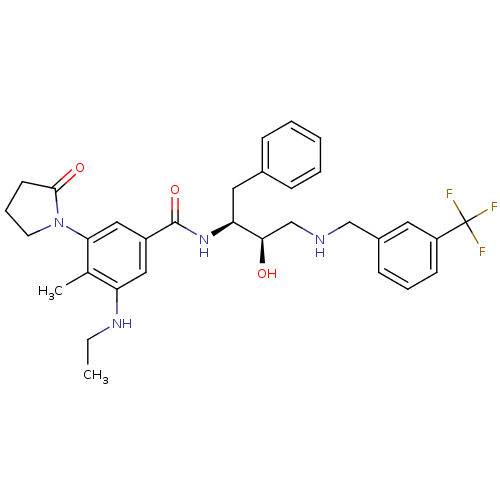 (hydroxyethylamine derivative, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26774 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-3-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |